Spravato for treatment-resistant depression
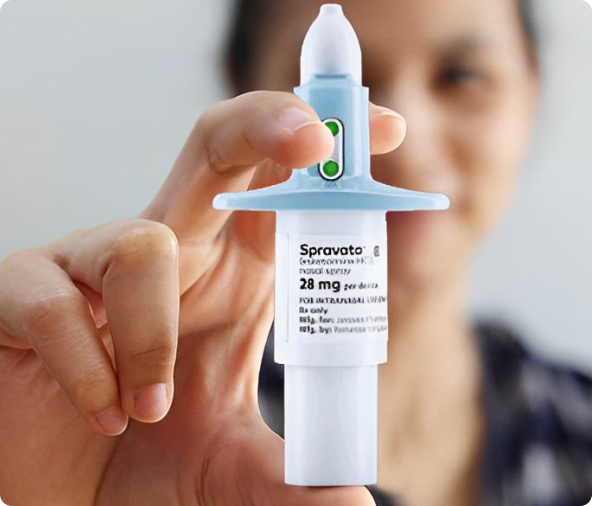
FDA-Approved for Use in Adults as a Single Form of Esketamine, SPRAVATO® is intended only for treating the following indications and cannot be used in combination to infuse study doses.
Treatment-resistant depression (TRD): For people who have deepening mental illness due to traditional antidepressants.
Severe Suicidal Thoughts & Behavior: Try antidepressants in people who have not responded to other MDD treatments.


As the first-of-its-kind, SPRAVATO® is a medicine that uniquely interacts with the NMDA receptor in the mind, offering a fresh approach to treating conditions when other well-being treatments have failed.

A dedicated team will be there to help you decide if SPRAVATO® is right for you. We conduct a thorough analysis, provide the necessary patient intake forms, and work closely with your Primary Care Physician or Pain Management Specialist, ensuring you receive the best possible care.
You must return to a treatment center for SPRAVATO® therapy – you will not be able to self-administer SPRAVATO® at home.
To learn more about our treatment center and how we can work together to provide you with SPRAVATO®, please contact Oak Hills Behavioral Health Solutions at 660-372-1313
For more information about SPRAVATO® or visit http://www.spravato.com
SPRAVATO® is a registered trademark of Johnson & Johnson and its affiliated companies.
© Janssen Pharmaceuticals, Inc. All rights reserved.
Located in Moberly, Missouri, our clinic is open six days a week and offers extended hours to accommodate your busy schedule.
We accept various insurance plans and offer a military discount to ensure that quality mental health care is accessible to all who need it.



© 2024 OAK HILLS. All Rights Reserved